How to Increase Engagement at Investigator Meetings
February 7, 2022 •Array Team

Conducting an effective clinical trial is more challenging than ever. Investigator meetings serve an important role in ensuring clinical trial sites are involved in discussions on implementation of complex protocols and provide a forum for training. A successful investigator meeting relies on engagement, communication, and follow-up support for effective study operations.
This blog will explore engagement strategies for successful investigator meetings and provide examples for you to use.
1. Identify key content for a focused event and set clear objectives .
Many investigator meetings suffer from being over ambitious in the amount of content to be covered.
Before the meeting, outline your critical goals and be selective with event content. Consult with internal study team colleagues, field teams and representatives of study sites to align objectives.
2. Choose the right event technology partner
While most pharma companies still prefer a face-to-face investigator meeting, today more are being conducted in virtual or hybrid formats. Rising costs, busy schedules and tight timelines make it important to maximize convenience. .png?width=500&name=Hybrid-VirtualView-NoText%20(1).png)
Due to travel restrictions, principal investigators and site staff expect virtual options. Virtual investigator meetings are cost-effective, highly convenient, and accessible.
Look for an event technology that supports hybrid and virtual events. The right platform paired with an intentionally designed presentation makes it easy to empower presenters, boost audience engagement, and measure the impact of your training.
3. Engage intentionally for learning retention
For some sites, it may be 3-6 months after the Investigator Meeting before they are recruiting or managing patients within a study protocol.
To maximize learning retention, investigator meetings should leverage active, engaged learning that centers on participation and discussion and aligns with endpoints of the study.
Engagement strategies can include:
- Polls/Surveys: To gauge opinions and understanding
- Q&As: To facilitate discussion with experts /study leadership
- Gamification: To encourage competition
- Saved slides/resources : To save important information and study resources
Incorrect responses to polling questions may indicate a need for follow-up with study site teams at site initiation or early monitoring visits.
4. Put inclusion and exclusion criteria into practice
Your clinical trial sites need to be able to select the appropriate patients. Familiarity with and understanding of inclusion and exclusion criteria is critical to optimize recruitment.
Rather than simply listing these criteria in a Death-by-Powerpoint presentation, consider a more active approach to provide opportunities for study teams to simulate patient selection/screening etc. in a risk-free learning environment.
Present a patient case and have them identify whether they’re a good candidate for the study. Providing feedback and the opportunity to review the case together will reinforce the training.
5. Invite feedback to identify and overcome challenges
Study site teams are great resources for information about what works and doesn’t work on the front lines. They can identify challenges in implementing the protocol locally, including patient availability, recruitment and retention, and new technologies
The investigator meeting provides an opportunity for seeking input on such challenges. Ask experienced teams what has worked well in similar studies: What tools and processes helped solve issues like low recruitment, administrative burden, and more?
Then open up a dialogue for site personnel to engage and collaborate. Be sure to build in pauses in presentations to allow space for responses. Share these takeaways in your post-event communications and clinical trial hub.
6. Leverage event data to extend support and resources
The key to successful clinical trials is extending support to boost adherence to study protocols.
Event tech like Array can capture individual engagement data. Here are a few ways clinical trial leaders can use event data from an investigator meeting.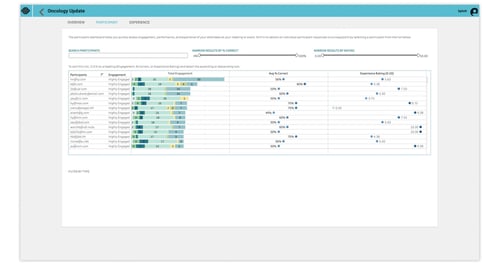
- Open-ended questions: Gather insights to find solutions to challenges and make crucial decisions on enrollment criteria before it’s finalized.
- Pre- and post-test results: See demonstration of learning and identify continued knowledge gaps
- Post-event evaluations: Capture feedback on certain roles and responsibilities, what areas they need more support resources, and attendee satisfaction.
- Saved content and notes: Track content engagement to identify most commonly saved content personalized learning inventories.
Make investigator meeting recordings, materials and tools, available on your clinical study hub. Provide useful resources for study site teams, as well as access for those unable to attend in person.
7. Learn from your history to improve
Have regular conversations with your sites during clinical research. After completion, review your past trials and identify root problems that led to poor performance. What knowledge, communication, and support gaps caused issues? What tools and materials did struggling sites lack?
As many pharma events are transformed by engagement and analytics, clinical trial leads and investigator meeting organizers can improve event management by applying lessons from past clinical trials to how they conduct investigator meetings in the future.
Conduct More Effective Investigator Meetings
To prevent investigator meetings from being long, expensive, and ineffective, event organizers and clinical trial leads need to work together. They need to collaborate with presenters and chairpersons to refine useful event content, maximize learning, and gather feedback.
With proper planning and engagement strategies, sponsor companies can see better data collection and improved site performance and relationships. Polls, surveys, and discussions are vital for investigator meetings to identify knowledge gaps and address challenges, like low enrollment.
Based on the strategies we’ve explored, leverage Array’s content engagement software to conduct worthwhile investigator meetings. With built-in tools to boost and gather engagements, as well as aggregate and individual event data for appropriate follow-up, Array enables sponsors and CROs to maximize the opportunity of an investigator meeting to support an efficient clinical trial.
See how Array can power more meaningful life science events →


